Matter and Atoms

"If, in some cataclysm, all of scientific knowledge were to be destroyed, and only one sentence passed on to the next generations of creatures, what statement would contain the most information in the fewest words? I believe it is that...all things are made of atoms."
-Richard P. Feynman, winner of the 1965 Nobel Prize in Physics
Matter is made of atoms, and atoms are comprised of protons, neutrons, and electrons.
Everything in the Universe is made of matter. Though matter exists in many different forms, each form is made out of the same basic constituents: small particles called atoms. Atoms themselves are made of smaller particles: protons, neutrons, and electrons. Protons and neutrons are composed of even smaller particles called quarks.
Scientists postulate that there are even smaller particles, but for the purpose of studying cosmology we will focus on the atom.
 [1.7a] Down the Rabbit Hole: Electric Charge
[1.7a] Down the Rabbit Hole: Electric Charge
Anatomy of an Atom
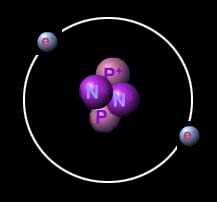
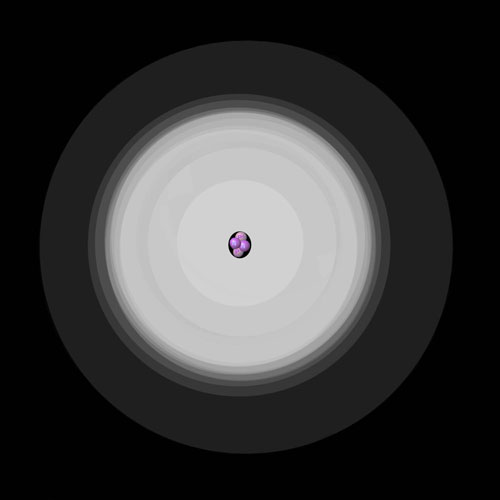
The classical orbit representation of the ground state of a Helium atom on the left showing the nucleus being orbited by two electrons in a distinct shell. On the right the Quantum Mechanical Model of the Helium ground state shows the 1s orbital with the same two electrons existing in regions of greater probability.
In the center of the atom, the strong force binds protons and neutrons tightly together forming the atom's nucleus. Electrons are found in the surrounding region, rapidly orbiting the nucleus. Classical mechanics describes the electrons as being held in distinct orbits by the electromagnetic force, very similar to the way gravity holds the moon in orbit around Earth. A more accurate model is given by quantum mechanics which describes the electrons as occupying regions of greater probability density called orbitals. On much larger scales, matter is attracted to other matter through the gravitational force.
 [1.7b] Down the Rabbit Hole: Quantum Mechanics
[1.7b] Down the Rabbit Hole: Quantum Mechanics
 [1.7c] Cosmic Conundrums: Matter
[1.7c] Cosmic Conundrums: Matter


